DATA INTEGRITY AND PRIVACY – COMPLIANCE WITH 21 CFR PART 11, SAAS/CLOUD, EU GDPR | Data integrity, 21 cfr part 11, Webinar

Reduce costs for compliance with data integrity: 21 CFR Part 11, SaaS/Cloud Tickets, Thu, Nov 17, 2022 at 1:00 PM | Eventbrite

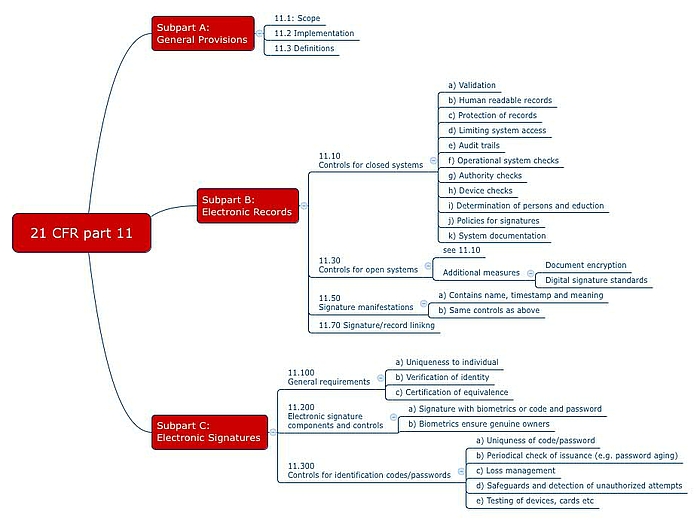
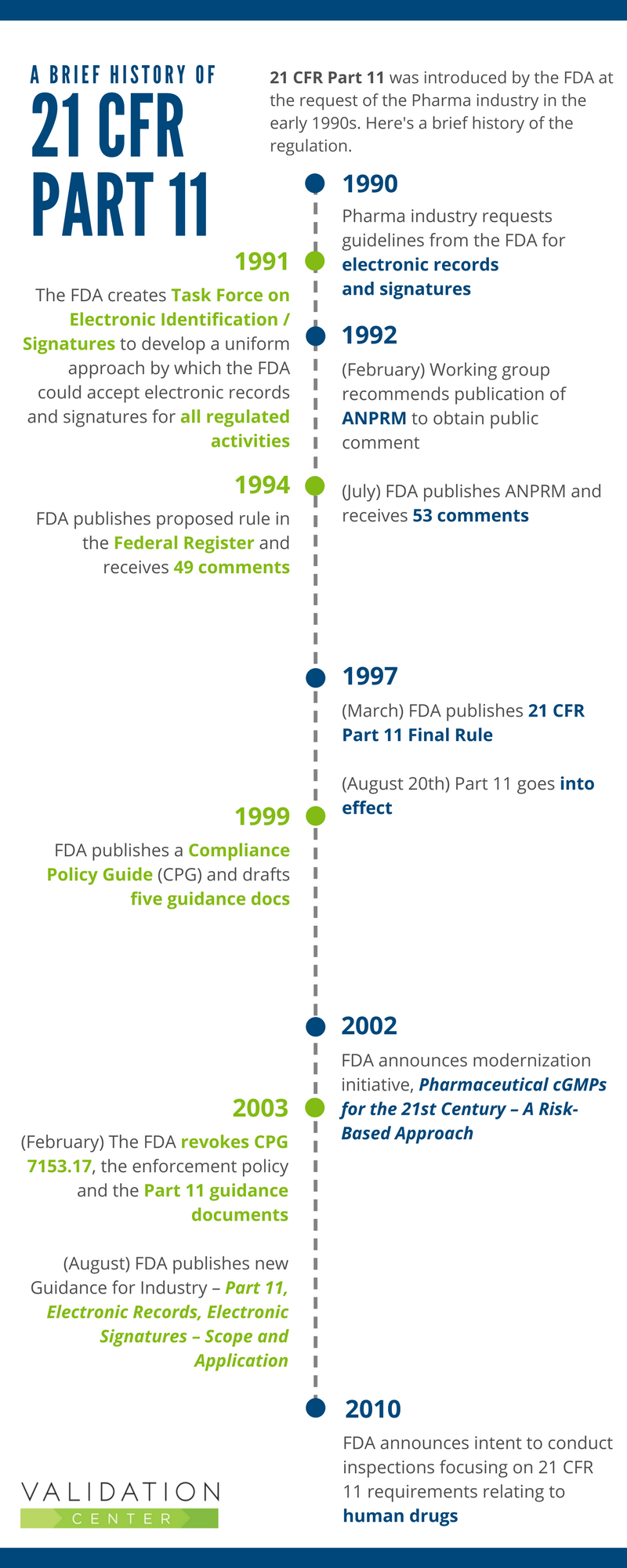
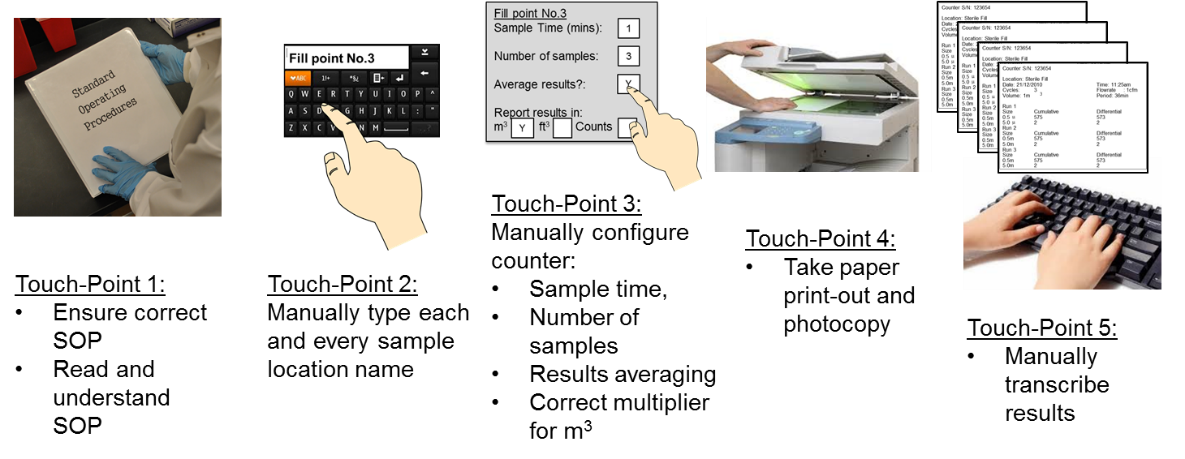
Clarifying and Meeting the Requirements of 21 CFR Part 11 and Data Integrity for Dissolution Testing | American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology