
OneClass: Part C. Water of hydration analysis 1. Formula of anhydrous salt used マテ (formula of th...

One mole of anhydrous salt AB dissolves in water and librates 21.0 J mol ^-1 of heat. The value of Δ H(hydration) of AB is - 29.4 J mol ^-1 . The

A treatise on the theory of solution including the phenomena of electrolysis . °6 and pass into the anhydrous salt andwater. Another hydrate, Na2S04. 7 HgO can be obtained byadding alcohol to
3 anhydrous salt to be 208.3 g. Using Equation 2, determine the number of moles of the anhydrous salt. Equation 2 Number of mol

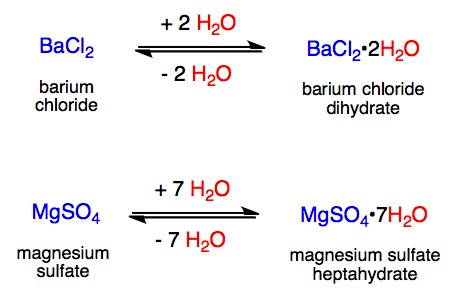







:max_bytes(150000):strip_icc()/anhydrous-chemistry-definition-603387_final-46e5c09532c045568b0a07dfddba7397.png)




.PNG)




